Chapter 7 worksheet 1 balancing chemical equations – Embarking on chapter 7 worksheet 1, the balancing of chemical equations emerges as a fundamental concept in chemistry. Understanding the intricacies of this process is crucial for comprehending chemical reactions and predicting the outcomes of various experiments.
This comprehensive guide will provide a step-by-step approach to balancing chemical equations, unraveling the significance of this skill in the realm of chemistry.
Introduction
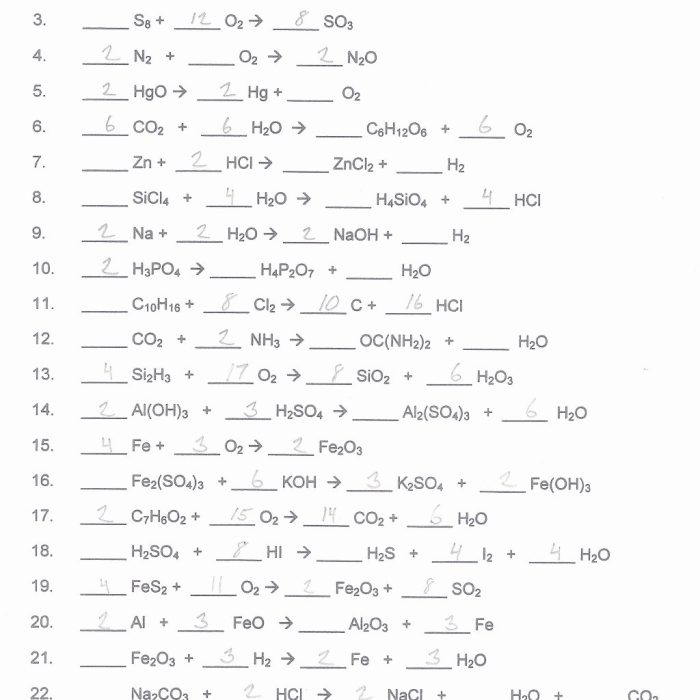
Balancing chemical equations involves adjusting the coefficients in front of chemical formulas to ensure that the number of atoms of each element is the same on both sides of the equation. It is crucial for accurate stoichiometric calculations and understanding chemical reactions.
By balancing equations, we can determine the exact amounts of reactants and products involved in a reaction, ensuring the conservation of mass and providing a basis for quantitative analysis.
Helpful Answers: Chapter 7 Worksheet 1 Balancing Chemical Equations
What is the purpose of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side of the equation matches the number of atoms of the same element on the products’ side. This adherence to the law of conservation of mass is crucial for accurate chemical analysis and predictions.
Why is balancing chemical equations important in chemistry?
Balancing chemical equations is pivotal in chemistry as it allows scientists to determine the stoichiometry of reactions. By understanding the mole ratios between reactants and products, chemists can calculate the exact quantities of substances required or produced in a given reaction.
What are some common mistakes to avoid when balancing chemical equations?
Common pitfalls to avoid include changing the chemical formulas of reactants or products, introducing unbalanced coefficients, and neglecting to account for all atoms in the equation. Careful attention to detail and a systematic approach are key to accurate equation balancing.